Manufacturing
Microneedles
Transdermal Patches
Research and Development Activities
Industry-Academia Collaboration
Intellectual Property
Manufacturing
About Manufacturing
An integrated process of sophisticated manufacturing technology and quality control
CosMED Pharmaceutical creates products under its own integrated development process from research and development to manufacturing.
In-house mold-forming technology allows us to achieve the reliable mass-production of dissolving microneedle products. CosMED Pharmaceutical has established its own manufacturing technology and quality control processes to develop highly advanced products from successes in transdermal therapeutic systems (TTS) research.

An Integrated Research, Planning, and Manufacturing Process
CosMED Pharmaceutical has created its own developmental process that integrates research, planning, and manufacturing.
The research & development division and manufacturing division coordinate in real time, adapting achievements from transdermal therapeutic systems (TTS) research and launching them to market as finished products. This sophisticated product development process is only possible thanks to CosMED Pharmaceutical being a research and development-based company.
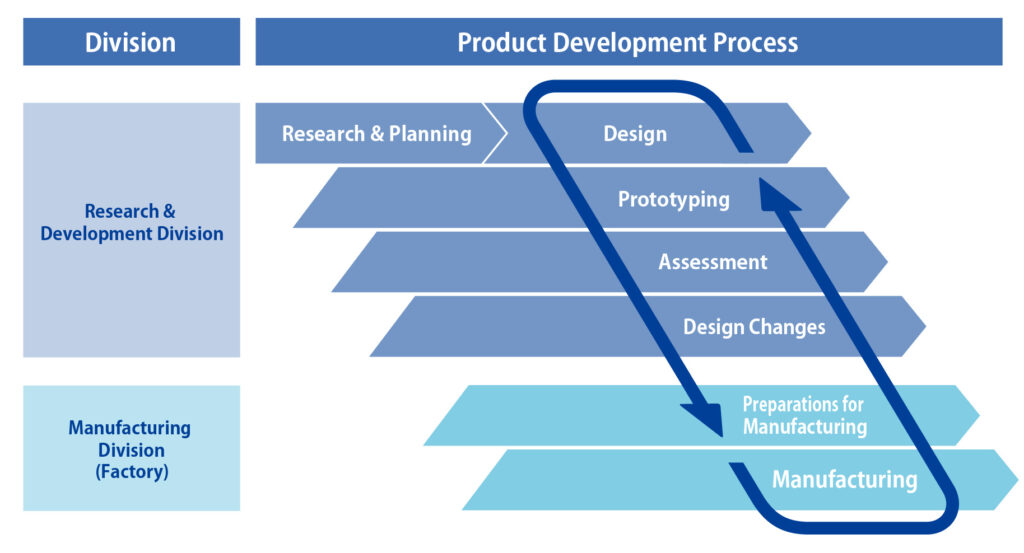
Sophisticated Development Process
Research, planning, and manufacturing are vertically integrated and operate in tandem.
Shorter developmental schedules, cost reductions, and quality improvements can be achieved with greater efficiency, and the development process can adapt quickly to market changes and customer demands.
A Diverse Range of Product Offerings
Starting from the initial planning stage, every division coordinates up to the final manufacturing stage in a one-stop process.
CosMED Pharmaceutical can respond flexibly to accommodate a diverse range of product designs and offer competitive products tailored to client and market needs.
Quality Control from the Research Stage
The product development process considers reliable supply and high quality from the research stage of development.
When a problem arises, quality control systems are in place to ensure early discovery of the cause and immediate amelioration, irrespective of where that problem occurs in the development process.
Manufacturing Factories
All three of CosMED Pharmaceutical’s manufacturing factories are located in Kyoto City. The factories have different capacities and roles, and all three coordinate to ensure the reliable production of high-quality products in Japan.
CosMED Pharmaceutical boasts an excellent track record in the manufacture of microneedle cosmetics, using high-level clean rooms in manufacturing factories with ISO 22716 certification (Cosmetics GMP). Every operation from manufacturing to packaging is undertaken with utmost care and attention.
Kyoto Factory


The Kyoto Factory is located alongside the research & development center and tasked with undertaking trial productions for new products, manufacturing operations for mass production, and technology testing.
● Cosmetics manufacturing: Licensed
● Cosmetics manufacturing and marketing: Licensed
● Quasi-drug manufacturing: Licensed
● Quasi-drug manufacturing and marketing: Licensed
● Medical device manufacturing: Registered
● Medical device manufacturing and marketing: Licensed
● Class 2 drugs manufacturing and marketing: Licensed
● ISO 13485 (Medical Devices): Certified
Katsura Factory


The Katsura Factory supports a wide range of microneedle production scales from small lots to large lots. The factory offers an integrated production process for adhesive products from solution production to coating, slitting, punching, and packaging. Delivers high quality products with efficiency by multi-tasking (multi-functional processing) with a small number of workers.
● Cosmetics manufacturing: Licensed
● Quasi-drug manufacturing: Licensed
● Medical device manufacturing: Registered
● ISO 22716 (Cosmetics GMP): Certified
● ISO 13485 (Medical Devices): Certified
Kissho Factory


As a “mother factory” for microneedle production at CosMED Pharmaceutical, the Kissho Factory features highly advanced technology and facilities and offers an integrated production process from solution production to needle manufacture, assembly, and packaging for the efficient production of high-quality products. The Kissho Factory also produces liquid cosmetics and medical devices for local anesthesia among other products.
● Cosmetics manufacturing: Licensed
● Quasi-drug manufacturing: Licensed
● Medical device manufacturing: Registered
● ISO 22716 (Cosmetics GMP): Certified
● ISO 13485 (Medical Devices): Certified
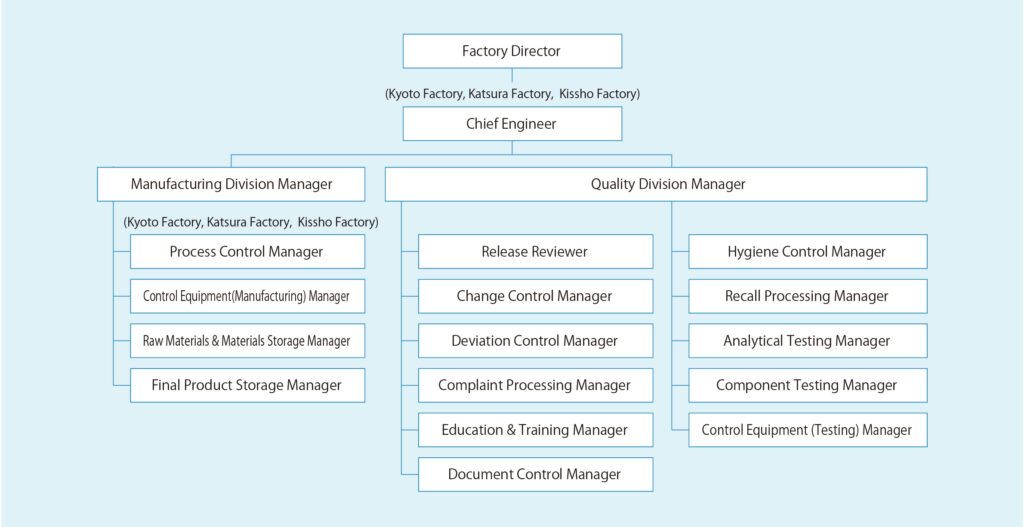
Manufacturing Controls
CosMED Pharmaceutical has established an integrated system of manufacturing controls at every factory to ensure operations progress smoothly on the manufacturing floor and product quality is assured.
Most notably, manufacturing controls for microneedle production require expert skills that are specific to microneedles, including formulation design and concentration controls for hyaluronic acid solutions, temperature and humidity controls for manufacturing operations, and mold controls. CosMED Pharmaceutical has established manufacturing control methods that ensure transdermal therapeutic system (TTS) products are mass-produced safely and reliably.

Quality Control and Assurance
With its quality pledge of “always in pursuit of the highest quality,” CosMED Pharmaceutical is committed to the ongoing improvement of product quality. CosMED Pharmaceutical has instituted systems to ensure quality throughout the manufacturing division and supports manufacturing in pursuit of the highest levels of quality assurance.
The Katsura Factory and Kissho Factory are both ISO 22716 (Cosmetics GMP) certified. The systems of quality control, quality assurance, and compliance at CosMED Pharmaceutical accord with international standards.
With the acquisition of ISO 13485 certification, the international standard for quality management systems in medical devices, CosMED Pharmaceutical will further reinforce its quality awareness across the organization, striving for continuous improvement and enhanced reliability.
We are committed to strengthening our supply chain for products in the medical and pharmaceutical domains.

Quality Control
CosMED Pharmaceutical is ISO 22716 (Cosmetics GMP) certified and its quality control systems accord with internationally recognized manufacturing conventions. Product safety is strictly controlled at every operation, from raw material testing to manufacturing and release to shipment. Ongoing quality improvements are also pursued through the introduction of new technologies and streamlining of manufacturing operations.
Quality Assurance
CosMED Pharmaceutical is licensed for the manufacture and marketing of pharmaceuticals, quasi-pharmaceutical products, and cosmetics, and implements strict controls based on GQP, GVP, and QMS ministerial ordinance*. CosMED Pharmaceutical has established a company-wide system of quality assurance and improves quality through regular internal audits and feedback. Complaints and quality issues are shared at monthly production meetings and management reviews are also led by management staff.
* GQP ordinance: Ministerial Ordinance on Standards for Quality Assurance for Drugs, Quasi-drugs, Cosmetics and Regenerative Medicine Products
* GVP ordinance: Ministerial Ordinance on Standards for Post-Marketing Safety Control of Pharmaceuticals, Quasi-Drugs, Cosmetics and Regenerative Medicine Products
* QMS ordinance: Ministerial Ordinance on Standards for Manufacturing Control and Quality Control of Medical Devices and In-Vitro Diagnostics